This work builds and open-source model of liquid solvent direct air capture technology to study the effects ambient environmental conditions have on its operating capacity. Results can be used to identify potential siting locations, and to support process design ideas to increase efficiency in cold climates.
Abstract
Emission trajectories produced by integrated assessment models increasingly suggest that gigatonnes of carbon removal will be required to stabilize atmospheric greenhouse gas concentrations at safe levels. This can be accomplished using the direct air capture of carbon dioxide, among other technologies. Process models of these systems assume that they would operate at standard ambient temperature and pressure, when capture rates vary with ambient conditions, including temperature, relative humidity, and other factors. Here, we build an open-source model of a liquid solvent direct air capture technology and analyze its capture performance as a function of hourly varying ambient environmental conditions across Canada. We find that, in the cool climate considered, capture performance is degraded due to both varying environmental conditions and the intermittent operation that could result. Our findings can be used to calibrate policy and investment decisions, and to support engineers in making operational design choices.
Similar content being viewed by others
Water management and heat integration in direct air capture systems

Introduction
To avert the worst consequences of climate change, we need to achieve more than the deep decarbonization of the energy system. An increasing number of emissions trajectories derived from integrated assessment models of the global climate and energy system also require substantial amounts of carbon removal to stabilize the climate at targets enshrined in the Paris Agreement1. Overshoot pathways acknowledge that mobilization towards a low-carbon system has been slow and might continue to be so, hence they envision varying numbers of years later in the century during which human activity generates net-negative emissions—in other words, when more carbon dioxide (CO2) is removed than emitted2. Carbon dioxide removal might also be required to offset emissions from energy services that could struggle to transition to low-carbon alternatives, or non-energy-based emissions (such as methane produced by livestock, or CO2 from cement production)—so-called hard-to-abate subsectors.
This potential need for large amounts of carbon dioxide removal has stimulated research into negative emissions technologies. Among this varied suite of natural and engineered systems is the direct air capture of CO2 (DAC). Existing literature has noted that DAC comes with high energy and capital requirements; our work is designed to provide a more informed weighting of DAC’s deployment potential relative to other mitigation options.
Typically, DAC is accomplished through the absorption or adsorption of CO2 into solvents or onto sorbents, respectively. Several DAC pilot or small-scale plants exist, and it is now approaching the commercial demonstration level of technology readiness for larger-scale plants, on the order of 102 to 103 ktCO2/year. Despite the technology’s relative novelty, the large carbon management infrastructure it requires3, and previous warnings about prematurely relying on it4, policymakers are increasingly expecting negative emissions technologies to contribute to decarbonization. For example, in its long-term strategy submission to the United Nations Framework Convention on Climate Change, Canada’s modelled approaches for achieving its legislated net-zero emission target could involve large amounts of DAC deployment, with upper bounds of 200 Megatonnes of CO2 (MtCO2) removal through DAC by 20505, depending on scenario. For context, Canada had 3 MtCO2 of operational capture capacity at the end of 20226; globally, 49 MtCO2 was captured in 2023, all from point sources of carbon emissions7.
Two leading DAC technologies are the solid-sorbent-based capture technology developed by Climeworks8, and the liquid-solvent-based capture technology developed by Carbon Engineering9. For example, the deployment of these technologies at the Megatonne scale, through the US Regional Direct Air Capture Hubs programme, will represent capture rates two orders of magnitude greater than any existing DAC facility10. In our model, we consider the latter technology, comprising a potassium hydroxide-based absorption system with a potassium loop and a calcium loop. In the potassium loop, atmospheric CO2 dissolves into the solvent, forming a carbonate. In the calcium loop, the carbonate ion is removed, regenerating the potassium hydroxide, and the CO2 is liberated and prepared for transmission and storage. The technology has been modelled and demonstrated9,11, and results of those models integrated into broader systems-level studies that consider DAC’s emergency deployment potential3, life cycle impacts12, and power system impacts13. However, little research considers how this technology’s operation in the real world would be greatly affected by climate. Ambient air temperature, pressure, humidity, and CO2 concentration all vary—some enormously and within short spans of time—and these variations will greatly affect capture rates and could occasion plant shutdown. This work complements similar analyses for solid-sorbent DAC by Cai14, Sendi15, and Terlouw16. For liquid-solvent DAC, An17 developed a model that is limited to a subset of temperature and humidity ranges, does not consider ambient pressure or CO2 concentrations, and cannot be replicated without proprietary software.
Here, we make several contributions to the literature on DAC’s potential role in the deep decarbonization of energy systems. One: we model the performance of a DAC plant in the real world, where ambient conditions greatly affect capture. Two: we extend this analysis across all of Canada, a jurisdiction that has expressed interest in deploying DAC at some of the largest scales. We develop estimates of the cost and performance penalties that climate conditions incur on DAC plants. Three: we develop an open-source chemical process model of liquid-solvent DAC (Methods). Unlike models built using expensive or proprietary programmes, ours can be employed or adapted to support investors, analysts, and policy makers.
Results
Process response to inlet conditions
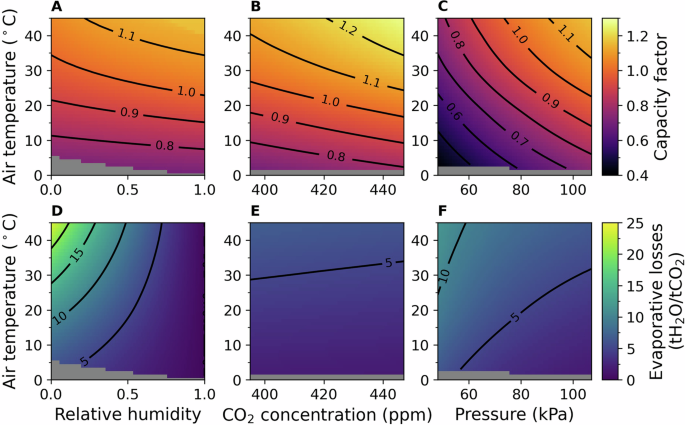
The performance of a nominal 1 MtCO2 gross capture facility (see supplementary information (SI) note S3 for plant specifications) was simulated for a range of possible ambient environmental conditions, modelled as inlet conditions at the capture unit (contactor). At nominal conditions, the model predicts the CO2 captured at 97% of the published predicted annual total (details in the SI, note S4)9. Conditions considered were ambient air temperature, pressure, humidity, CO2 concentration, and liquid solvent temperature. The range of conditions simulated is tabulated in the SI (Table S7). We consider evaporative losses, as the large volumes of air processed through the contactor (approximately 57,000 m3 s−1) are free to absorb moisture from the aqueous solvent. As the contactor is designed to maximize surface area for the absorption of CO2, so too does it act as a very efficient evaporator. Makeup water is required to replace these evaporative losses. Performance of the plant in terms of capacity factor (CF) (1) and evaporative losses (2) is summarized in Fig. 1. The power required per tCO2 captured is largely insensitive to environmental conditions, with the exception of fan work at the contactor, where lower air densities require higher volume flow rates to achieve the specified mass flow rate. Performance in terms of the energy intensity of capture are summarized in SI Fig. 8. The liquid inlet temperature is assumed to be at equilibrium with the ambient air temperature. Inlet (solvent and air) temperatures at or below 0 °C are not simulated, as we model the thermodynamic properties of the solvent as pure water, and these temperatures represent freezing conditions (KOH and K2CO3 in solution are expected to depress the solvent freezing temperature by approximately 3 °C). Engineering solutions can enable operation in sub-zero temperatures—these include active heating of the inlet air. However, this would be a large additional process load due to the dilute concentrations of CO2. For example, air at 0.06% CO2 by mass, assuming a 100% capture rate, requires the heating of 99.94 t-air per tCO2. If we assume a heat capacity of 1 kJ kg−1 K−1, this represents a heating load of approximately 0.03 MWh tCO2−1 per K heated.
Over the range of conditions simulated, we see the greatest change in capture rates over the ambient temperature range, followed by pressure, CO2 concentrations, and lastly humidity. Higher ambient temperatures increase reaction kinetics, modelled via correlations for the reaction rate constant, ion diffusivity, liquid-phase mass-transfer coefficient, and the CO2 reference solubility for use in Henry’s Law. Higher pressures and CO2 concentrations both increase the CO2 partial pressure, which proportionally increases the interface concentration (9), directly increasing the capture rate (7). We see the least change in capture rates over the humidity range, as humidity only indirectly affects capture rate by lowering or raising the liquid temperature via evaporation and condensation, respectively. Evaporation rates are modelled as driven by the difference of the humidity ratio and saturated humidity ratio at the liquid temperature (see Methods), and as such are driven strongly by both humidity and temperature. As warm air can absorb much more moisture than cold air, the highest evaporation rates are when the air is both dry and warm. Pressure has a milder influence on evaporation, with increasing pressure decreasing the saturated vapour pressure (SI equation S12). CO2 concentrations have no direct effect on evaporation, only lowering the evaporative losses when normalized by the CO2 capture rate.
CF is shown at different ambient air temperatures and A relative humidity; B CO2 concentration; and C pressure. Similarly, evaporative losses are shown at different ambient air temperatures and D relative humidity; E CO2 concentration; and F pressure. Where not specified, inlet conditions comprise relative humidity of 0.63, CO2 concentration of 400 ppm, and pressure of 100 kPa. Masked cells represent freezing conditions due to evaporative cooling.
Regional variations affect overall plant performance
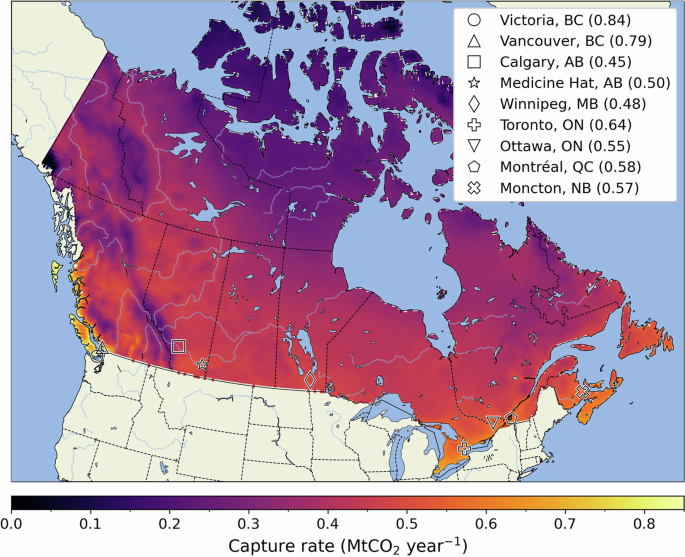
We use hourly climate data to predict changes in performance for the same 1 MtCO2 plant as a function of differentiated and variable climate conditions. Regional environmental profiles influence capture rates, evaporative losses, and the intermittency of plant operation. Contactor operation requires air temperatures above freezing, leading to strong seasonal intermittency in operation within the temperate latitudes examined. Regional differences in capture rates across Canada are shown in Fig. 2, with energy use and evaporative losses shown in the SI (Figs. S3 and S4, respectively). With respect to CO2 capture, we see the expected decrease in capture with latitude, as average temperatures decrease, although regional geographic features can dominate a microclimate. For example, on the west coast, the ocean has a strong regional effect in moderating winter temperatures, and here we find the highest annual capacity factors (over 0.8) in the country (this effect is less pronounced on the east coast, due to the colder Labrador current). However, adjacent to the Pacific coast, we find some of the least-suitable climes for DAC (CF near 0.2), among the low temperatures and high altitudes of the Coast Mountains and Rocky Mountains.
Locations discussed here are representative of Canada’s most prevalent climate regions, as well as a diversity of its electricity pricing and grid emission intensities. Capacity factors for the selected locations are given in brackets. White areas fall outside the study region.
Evaporative losses are highest over the Prairies, which is important as this region also represents the highest solar resource potential in Canada. However, it is also Canada’s breadbasket, and competition for water resources by DAC is an additional cost of deployment. Solar resources, which could provide a low-cost, low-emission power source for DAC, are often correlated with existing water-stress. Of the locations identified in Fig. 2, the lowest annual evaporative losses occur in Toronto, Ontario (ON) (1.84 tH2O tCO2−1), and the highest in Medicine Hat, Alberta (AB) (4.51 tH2O tCO2−1). The energy intensity of capture has little regional variation, with Fig. 2 locations representing a range of 1.80 to 1.87 MWh tCO2−1. The highest energy use within the study region (2.06 MWh tCO2−1) occurs over extreme elevation, where low pressures increase contactor fan and CO2 compression loads.
Plant operation will be dynamic, potentially intermittent
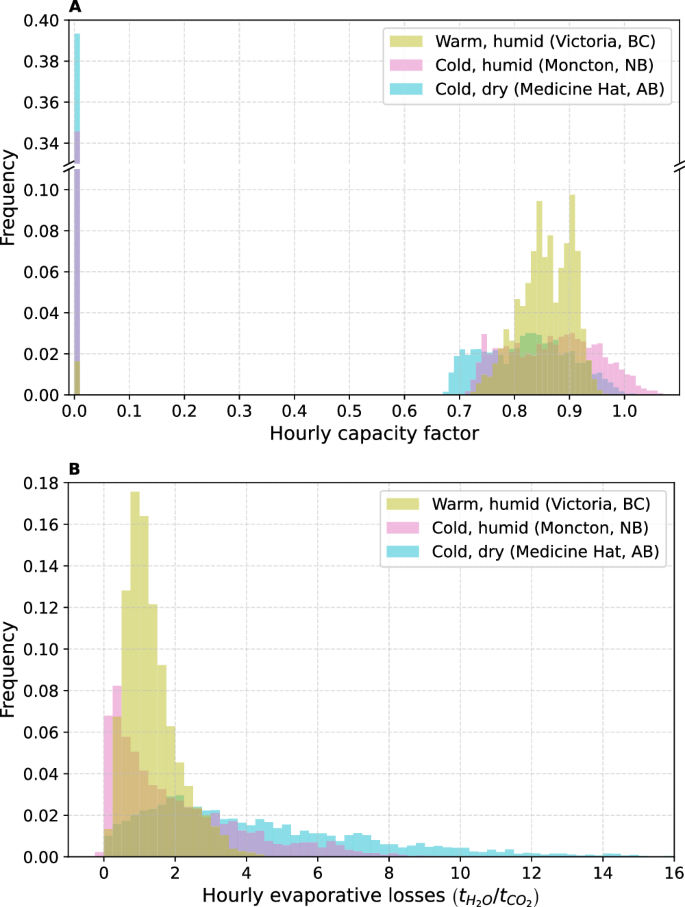
Varying ambient conditions will necessitate dynamic DAC plant operation and control. We analyze plant performance in locations that are representative of the most prevalent climate regions studied, outside of polar and sub-arctic climes that include the Northern Territories. These archetypal locations include Victoria, British Columbia (BC), Medicine Hat, AB, and Moncton, New Brunswick (NB), representative of warm-humid, cold-dry, and cold-humid climes, respectively. Figure 3 shows the annual distribution of hourly capacity factors and evaporative losses that would be expected from DAC plants deployed at each of the three locations. Annual performance summaries, as well as climate classification of the regions within the Koppen climate classification scheme, are given in the SI (Table S8).
Frequencies are normalized as fractions of the year; the balance of the frequencies are when the plant is considered inoperative (i.e. temperatures are below freezing). The bi-modal distribution for A hourly capacity factors is a function of the seasonal distribution of warmer, daylit hours, see SI Figure S7. B Evaporative losses yield a distribution with a long tail in colder, drier regions like Alberta.
The capacity factors shown in Figs. 2 and 3 are an upper bound: they represent the sum of the hourly capture as a function of varying environmental conditions, relative to 1 MtCO2 of annual capture. In other words, they ignore the feasibility of starting up and shutting down a large and complex chemical plant on hourly timescales, when plant startup in process industries could take hours to weeks. In the temperate latitudes considered, there are typically times of the year when air temperatures fluctuate around 0 °C, so that air temperatures are only intermittently suitable for plant operations.
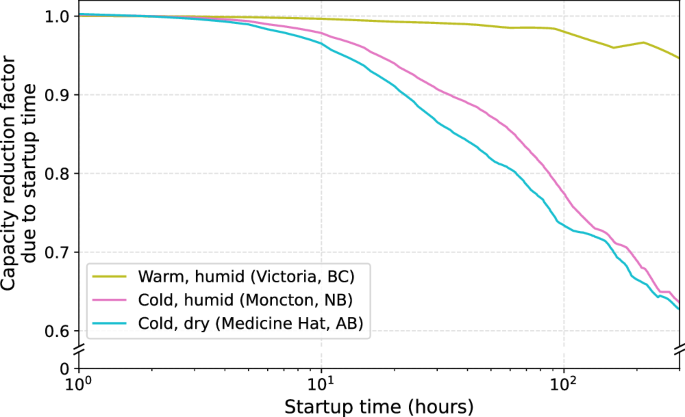
In Fig. 4, the effect of intermittency on performance is explored by incorporating a plant startup time: the time it would take to restart the plant after shutting it down to prevent the solvent from freezing. A startup time of 1 hour represents our maximum capacity factor, since we use hourly climate data in calculating earlier capacity factors. The ordinate, the capacity reduction factor, is the ratio of the capture capacity—reduced by startup time—to the maximum capture capacity. The capacity reduction factors in Fig. 4 further emphasize the importance of ambient conditions to liquid-solvent DAC plant siting. For example, a startup time of 100 hours—or roughly four days—could de-rate plant performance by 5–25%. Calciner startup, including preheating, may provide a minimum startup time of approximately 10 hours18,19. However, these plants comprise many tightly coupled units, including heat exchangers. Start-up times could therefore exceed this.
The curves represent the capacity factor as a function of startup time for each of the selected regions, normalized by the capacity factor before reductions from plant startup. This data is smoothed; the unsmoothed plot is given in the SI (Figure S5).
The feasibility of avoiding shutdown by pre-heating the air was estimated above at 0.03 MWh tCO2−1 K−1. Heating the solvent represents a lower energy demand, with approximately four times the heat capacity of air, but one-eighth of the mass flow rate. However, strong evaporative cooling would require heating of the solvent well beyond the freezing point. For example, at nominal humidity and pressure, inlet air at –1 °C would require heating of the solvent to 7 °C to avoid freezing. While these represent large loads for active heating, this could be an opportunity for passive solar or low-quality geothermal heat.
Levelized cost of CO2 captured from the atmosphere
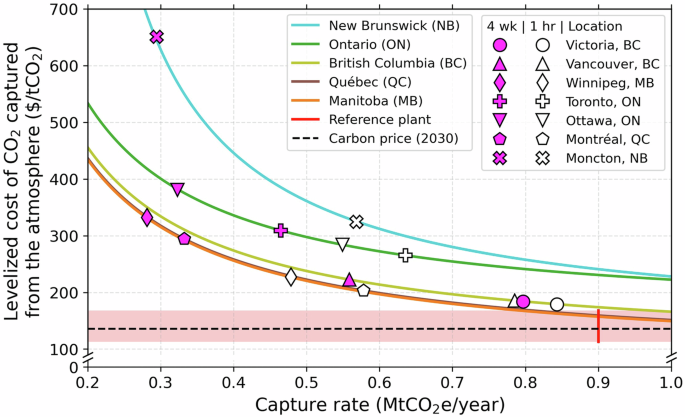
A levelized cost of CO2 captured from the atmosphere (LCOR) is estimated based on plant capital costs (CapEx) of 1 billion US dollars (USD)9, provincial electricity prices for large industrial consumers20, average provincial electric grid carbon intensities21, capacity factors, and location-specific energy intensities of capture (see SI Tables S8–S9). The LCOR does not include costs or emissions associated with the transportation or storage of the captured CO2 but does include the energy required for compression to typical pipeline pressures. Embodied emissions from the construction of the plant have not been included but are estimated elsewhere at 0.00088 tCO2 tCO2-captured−1 22. A discount rate of 5 per cent and a capital amortization period of 25 years allow for comparisons of cost estimates with9. The procedure for calculating LCOR are given in the methods ((32)–(36)). The LCOR for a plant with a 1-hour versus 4-week startup time is calculated. The resulting LCOR are shown in Fig. 5. All prices are given in USD.
Markers represent the LCOR based on the actual CF of the specific locations identified within the provinces. White markers are representative of a startup time of 1 hour, and magenta markers 4 weeks. The shaded region represents estimates from prior work modelling a plant operating at nominal conditions and 90% utilization. Not all locations in Fig. 2 are shown here, as the Alberta provincial grid intensity is such that emissions would exceed capture, and so the corresponding LCOR is undefined.
We assume that the DAC plant is wholly electrified. We have made the simplifying assumption that the capital cost of an electrified plant is equivalent to the reference process, at 1B USD. In modelling the operational expenses, we restrict ourselves to the energy requirements of the five main process steps (see Methods): absorption, precipitation, calcination, slaking, and compression (other operational or maintenance costs are ignored). Regarding the reference process and since the plant is assumed to be electrified, we do not model the power island, CO2 absorber (on turbine exhaust), oxygen plant (air supply unit), or the combined auxiliary loads comprising 2.6 MW. As discussed elsewhere22, indirectly heated calciners suitable for electric resistance heating are commercially available, but not at the scale considered here, and would require a dedicated fluidizing gas. At the nominal operating conditions, the capture energy is predicted to be 1.80 MWh tCO2−1. At this capture energy, an electric grid carbon intensity of 555 gCO2kWh−1 yields no net capture of CO2. Therefore, it would be counterproductive to use grid electricity for DAC in Nova Scotia, Saskatchewan, or Alberta, since electric grid carbon intensities in those provinces exceed this threshold due to their overwhelming reliance on fossil fuels—something that is peculiar in the Canadian context, where electric power grids are relatively cleaner than they are elsewhere in the world.
We see a range of LCOR values in Canada, with the most favourable regions yielding values of approximately 190 $ tCO2−1. This can be compared to estimates for nominal operating conditions at 90% utilization of 113–168 $ tCO2−19. Inexpensive and low-carbon electricity, such as in Québec, can result in regions with low capacity factors having a competitive LCOR compared to regions with more favourable climate conditions, such as British Columbia. These prices can also be compared to the (Canadian) Federal Government’s projected 2030 price on carbon of 134 $ tCO2−1 (170 CAD tCO2−1)23. A map of the regional distribution of pricing for Canadian provinces is included in the SI, Fig. S10.
Discussion
Jurisdictions considering large levels of future carbon removal through direct air capture must decide where these chemical process facilities will be sited. In addition to considerations regarding transportation, storage, and social acceptance, these facilities will contend with a more fundamental technical challenge: their capture rates (and thus their carbon removal costs) are going to depend on ambient environmental conditions like temperature, relative humidity, and pressure, among others. Across most of Canada, seasonal freezing temperatures will result in lower capacity factors for liquid solvent DAC technologies. Where the climate is amenable to such a process, the availability of low-carbon energy must also be considered, as net carbon removal is the measure of success. Combining regional climate profiles, energy emissions intensities, and energy prices, we can offer an initial estimate of region-specific carbon removal costs. For jurisdictions that wish to build liquid solvent DAC plants within their borders, these estimates can be used to calibrate policy and investment decisions. Policy makers can consider our results in calibrating the contribution of DAC to their net-zero emission plans, as well as the size of proposed incentives. In addition, policymakers and investors can use our results to guide early investments in CO2 transportation and storage infrastructure.
Our results show that, for temperate climes, plant startup times should be kept short. Unlike electric power plants, chemical process plants may need anywhere from hours to days to restart, depending on the design and integration of their units. Designing-in protocols for rapid startup that accommodate intermittent operation would be a prudent step on the part of engineers. Arwa demonstrates a method for decoupling the capture and recovery loops for better integration of liquid-solvent DAC with intermittent renewable generation24. This approach could also have benefits for climate-driven intermittent capture.
Energy system modellers must represent complex chemical processes with greater fidelity in their models, to better understand the opportunities and challenges associated with these processes in the energy transition. Ignoring ambient conditions or intermittent operation could have profound implications on energy system operations, including power system reliability, especially as more chemical processes are electrified or converted to electrofuels that are also dependent on electricity for their synthesis.
Our cost and performance estimates should be considered as lower bounds. Future demands for labour and materials, not to mention the energy required for carbon removal will be global in scale, meaning that DAC plant deployment in any nation will not only compete with deployment in other jurisdictions but also with concurrent mitigation efforts in all sectors of the economy. It should also be considered that there is no full-scale trial data with which to validate these predictions, and the model should be reconsidered as these data become available.
Finally, our results focus on one (albeit more technologically mature) liquid solvent DAC technology. The capture model can be readily adapted to alternative aqueous liquid solvents through the inclusion of the appropriate chemical-specific correlations, specifically the salt-effect parameter, reaction rate constant, and ion diffusivity. Solid sorbent DAC systems will behave differently across ambient conditions; their performance is also dependent on the sorbent used. It may be that liquid-solvent and solid-sorbent DAC develop as complementary technologies, as they favour warm-humid and cold-dry15 climates, respectively. Methods for the intermittent operation of solid sorbent DAC plants could also differ from those for liquid solvent systems. Investors and policy makers should consider these differences as they make decisions regarding the deployment of a portfolio of carbon removal technologies in pursuit of net-zero emission targets.
Methods
Chemical process
We develop an open-source model in the Python programming language of a potassium hydroxide-based liquid-solvent DAC process described elsewhere9. There are five main process steps. One: dissolution and binding of atmospheric carbon dioxide (CO2) within the contactor, in the form of potassium carbonate (K2CO3) (3). Two: precipitation of calcium carbonate (CaCO3) within the pellet reactor (4). Three: liberation of CO2 within the calciner (5). Four: hydration of quicklime (CaO) to slaked lime (Ca(OH)2) within the lime slaker (6). Five: compression of the concentrated CO2 for transport and utilization or storage. Process modelling is limited to the contactor, which, when combined with the inlet flow conditions, determines both the capture rate of the facility and the evaporative losses. Mass and energy balances are performed for the main process steps to generate estimates of the plant’s mechanical and thermal energy loads. Thermal and mechanical loads are treated as equivalent from a load perspective, when estimating the total power requirements. Heat integration is assumed at 50%, with the manner of energy recovery not considered. Energy recovered from heat integration is discounted directly from the power load.
Modelling the contactor
The contactor is modelled as a 1-dimensional counter-flow packed column. Ambient air at mass flow rate m˙G enters at the bottom of the contactor and is forced through the column, while the liquid solvent at mass flow rate m˙L enters at the top. The column is filled with random or structured packing to increase the specific surface area available for mass transfer a, without excessive pressure drop across the column.
CO2 capture process
Whitman’s film theory25, 26 is used to predict the gas-to-liquid CO2 mass transfer rate JL,CO2(7), which is the product of the liquid-phase mass transfer coefficient kL(8), estimated by Onda’s correlation27, the interface concentration ci,CO2(9), determined using Henry’s Law for solutions, and the enhancement factor E ((10)–(12))25. In finding kL, additional correlations for the effective packing diameter dp, wetted area aw, and diffusivity D are used (these are given in SI Table S4). Other terms in the equations below include the kinematic viscosity ν, acceleration due to gravity g, and liquid superficial velocity Us.
To solve for E requires: (a) the second-order rate constant k2 for the reaction of CO2 and KOH, with correlations elaborated by Pinsent28 and Kucka29, and included in SI Table S4; (b) the diffusivity of liquid-phase CO2 DL,CO2 as correlated by Wilke & Chang25, which can also be found in Table S4; (c) the diffusivity of the binding species (OH−) DB, as correlated by See30 for temperatures 1≤TL≤25 °C, and the Stokes-Einstein method31 for TL >25 °C; and (d) the stoichiometric coefficient z of the binding species (in this case 2, see equation (3)), where Ei is (12) the enhancement factor for an instantaneous reaction.
The Henry’s Law constant H is a function of liquid temperature and ionic strength (13). The reference solubility of CO2 in pure water H0(T) is predicted using the Van’t Hoff extrapolation32(14), where the reference temperature T0 = 298.15 K, H0(T0) = 3.4 × 10−2 atm L mol−1, the enthalpy of solution ΔsolH = − 19.95 kJ mol−133, and R is the molar gas constant. Following the method of van Krevelen and Hoftikzer25, H in solution is correlated to H0 by the ionic strength I(15) and Setchenov salt-effect parameter h(16), where ci is the concentration of ions in solution with charge zi, and h+, h−, and hCO2 are the salt-effect parameters for the positive ions, negative ions, and dissolved CO2 in solution, respectively.
To predict the total capture rate, the column is discretized into n finite volumes spanning the diameter of the column, of volume dV. Conditions are modelled as steady within each volume. For each volume i, the mass capture rate m˙CO2,i is the product of JL,CO2; the area available for capture Ac(17); and the molar mass of CO2 MCO2. The packing efficiency, ηpack, accounts for the incomplete wetting of the packing at low solvent flow rates (we use the value provided by Holmes and Keith of 0.8011).
Evaporation and condensation
Evaporation and condensation rates m˙vap(20), with associated heat transfer, are also estimated for each volume, following the method described by Sutherland34, where the evaporation rate is driven by the difference in the air humidity ratio ω and the saturated humidity ratio ωsat at the liquid temperature. The convective mass-transfer coefficient ϕ is predicted by an empirical correlation from cooling tower data described by Kuehn (21)35, where c and n are coefficients fitted to the specific column under analysis, within the range 1.0≤c≤1.7 and 0.25≤n≤1.0, and V is the total column volume. In the absence of experimental data, the recommended values35 of c = 1.3 and n = 0.6 are used.
Sensible and latent heat transfer occurs between the gas and liquid phases within the column, with the packing and column walls modelled as adiabatic. Energy released from the reaction of CO2 with KOH is ignored: as the liquid molar flow rate is 2-3 orders of magnitude greater than the reaction rate, the resulting liquid temperature changes are negligible ( ≈ 2 × 10−3 K at 20 °C). Thermodynamic properties of the gas and liquid phases are modelled as moist air and water, respectively. Convective heat transfer rates are correlated to evaporation rates using the Lewis number Le (22), sometimes referred to in this form as the Lewis relation, which is the ratio of the convective heat-transfer coefficient ζ to the mass-transfer coefficient ϕ, normalized by the specific heat capacity of moist air cm. For air and water vapour mixtures near atmospheric pressures Le = 0.936.
Convective heat transfer based on sensible heat difference is exchanged between the gas and liquid phases (23), while latent heat due to vaporization and condensation is exchanged with the liquid phase (24), where hvap (J kg−1) is the enthalpy of water vapour at the temperature of the liquid. The temperature change dT ((25), (26)) is found from the change in enthalpies (dhG, dhL) and the specific heat capacities (cm, cL) of the gas and liquid phases, respectively. Note, hL is defined as joules per kg of dry air. A diagram of the heat and mass transfer across a single column element is given in the SI (Figure S1).
Solution method
The model comprises a steady-state solution for heat transfer and H2O and CO2 mass transfer between the gas- and liquid-phases within the contactor. To arrive at the solution, the column is discretized spatially into a 1-dimensional array of volume elements between the inlet and outlet. Evaporation and heat exchange rates for each element are solved using the forward Euler method, which iterates until convergence. The evaporation solution is used as the input to find the CO2 absorption rates, which are similarly solved using an iterative backward Euler method. A schematic of the element-wise energy and mass balances are given in SI Fig. S1, and of the overall solution procedure in SI Fig. S2. The code is written in Python and published under an open license via CC BY-NC-SA 4.0.
Power consumption
Estimates of contactor power consumption are based on solvent pumping and fan work. In both cases, the power P is a function of the volumetric flow rate Q, pressure drop Δp, and efficiency η(27). In the case of solvent pumping, the pressure drop ΔpL is modelled as the hydrostatic pressure loss over the height of the contactor H(28). In the case of fan work, a pressure drop ΔpG correlation is provided by the vendor of the packing modelled for this study11, where Z is the depth of the packing and Us is the superficial gas velocity (29). While generalized pressure drop correlations are available37, they are fitted to higher flow-rate data, and over-estimate the pressure drop for this relatively low flow-rate application. The total solvent pumping efficiency ηpump(31) is the product of the driver efficiency ηd and the intrinsic efficiency ηi(30)25.
Pellet reactor
Transfer of carbon from the potassium loop to the calcium loop through precipitation as CaCO3 takes place within a recirculating, fluidized-bed pellet reactor. The bed height hbed, fluidization velocity Umf, and average pellet diameter dp are parameters taken from the nominal plant as described elsewhere9. Similar to the contactor, the energy required is estimated based on the pump work. The pressure drop Δpbed is the balance of the drag force with the gravitational force on the particles38. Void fraction modelling is described in the SI (note S1). The total pump efficiency is calculated as for the contactor. The total CaCO3 fines-to-disposal or makeup rate is assumed to be 1% of the CaCO3 produced9.
Calciner
Energy for calcination is modelled as the sum of the thermodynamic energy of the calcination reaction, 178.3 kJ mol−19, and the energy required to raise the sensible heat of the CaCO3 input stream to the reaction temperature of 900 °C, having a specific heat of 834.3 J kg−1 K−1. Thermal and conversion efficiencies of 78% and 98%, respectively, are taken from ref. 9.
Lime slaker
Quicklime from the calciner is hydrated to slaked lime within the lime slaker. Mechanical energy consumption is estimated at 17 kWh per tonne of quicklime39. Conversion efficiency is assumed at 85%9, producing thermal energy at 63.9 kJ mol-converted−1. Additional thermal energy is available from reducing the sensible heat of the input CaO stream from 900 to 300 °C. We assume that the thermal energy produced is integrated into plant operations by a heat integration factor ηhi = 0.5.
CO2 compression
The concentrated CO2 stream is cooled and compressed for transport and subsequent utilization or storage. Thermal energy is released through reducing the sensible heat of the CO2 stream from 900 to 40 °C. Similar to the lime slaker, we assume that this energy is integrated at ηhi = 0.5. To estimate the mechanical power required, compression is modelled as isothermal (see SI note S2). In this study, the outlet temperature and pressures are assumed 40 °C and 15.1 MPa, respectively.
Inlet air physical properties
These, comprising temperature, pressure, humidity, and CO2 concentration, are determined by ambient environmental conditions at the plant site. We consider hourly environmental conditions throughout Canada in 2019. Hourly surface air temperature, relative humidity, and pressure data are taken from the fifth major global reanalysis produced by the European Centre for Medium-Range Weather Forecasts40. CO2 levels (available at three-hour intervals) are taken from the Copernicus Atmosphere Monitoring Service global greenhouse gas reanalysis41. We conduct the analysis for Canada; grid cells entirely over water are not considered. For each cell, performance is predicted hourly over the sample year of 2019. Spatial and temporal modelling resolutions correspond to the resolutions of the European Centre for Medium-Range Weather Forecasts’ dataset.
Levelized cost of CO2 captured from the atmosphere
A levelized cost of CO2 captured from the atmosphere (LCOR) (36) is estimated based on plant capital costs (CapEx) of 1B USD9, regional electricity prices for industrial consumers20 (EP), average provincial grid emission (GE) carbon intensities (CI)21, annual capacity factor (CF), and location-specific energy intensity of capture (all prices are given in US dollars (USD)). A discount rate r = 0.05 and a capital amortization period of n = 25 years give a capital recovery factor (CRF) of 0.071 (32), which allows comparisons of cost estimates with Keith at CRF = 0.0759. Regional pricing, grid emission intensity, and the resulting LCOR are summarized in SI Table S9 and presented in Fig. 5.
Code availability
The code is published under an open license via CC BY-NC-SA 4.0. It can be accessed at https://github.com/APEX-Carleton/Liquid-solvent-DAC/releases/tag/v1.0.0. Model output, used to generate Fig. 1 and Supplementary Fig. S8, are available at the following https://doi.org/10.5281/zenodo.13684667.
References
-
Beuttler, C., Charles, L. & Wurzbacher, J. The role of direct air capture in mitigation of anthropogenic greenhouse gas emissions. Front. Clim. 1, 469555 (2019).
-
Abdulla, A. et al. Atmospheric verification of emissions reductions on paths to deep decarbonization. Environ. Res. Lett. 18, 044003 (2023).
-
Hanna, R., Abdulla, A., Xu, Y. & Victor, D. G. Emergency deployment of direct air capture as a response to the climate crisis. Nat. Commun. 12, 368 (2021).
-
Fuss, S. et al. Betting on negative emissions. Nat. Clim. Change 4, 850–853 (2014).
-
Environment and Climate Change Canada. Exploring approaches for Canada’s transition to net-zero emissions: Canada’s long-term strategy submission to the United Nations Framework Convention on Climate Change. https://unfccc.int/sites/default/files/resource/LTS20Full20Draft_Final20version_oct31.pdf. Accessed: 2024-1-2.
-
Canada Energy Regulator. Market snapshot: New projects in Alberta could add significant carbon storage capacity by 2030. https://www.cer-rec.gc.ca/en/data-analysis/energy-markets/market-snapshots/2022/market-snapshot-new-projects-alberta-could-add-significant-carbon-storage-capacity-2030.html. Accessed: 2024-1-2.
-
Global CCS Institute. Global status of ccs 2023. https://www.globalccsinstitute.com/wp-content/uploads/2023/12/Global-Status-Report-2023_Slide-Deck_Americas-EMEA-Website.pdf. Accessed: 2024-1-2.
-
Pretorius, F. Methodology for direct air capture. https://climeworks.com/news/methodology-for-permanent-carbon-removal (2022).
-
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
-
Smith, S. M. et al. The state of carbon dioxide removal 2024 – 2nd Edition. https://www.stateofcdr.org (2024).
-
Holmes, G. & Keith, D. W. An air-liquid contactor for large-scale capture of CO2 from air. Philos. Trans. R. Soc. Lond. Ser. A: Math. Phys. Eng. Sci. 370, 4380–4403 (2012).
-
Deutz, S. & Bardow, A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat. Energy 6, 203–213 (2021).
-
Bistline, J. E. T. & Blanford, G. J. Impact of carbon dioxide removal technologies on deep decarbonization of the electric power sector. Nat. Commun. 12, 3732 (2021).
-
Cai, X., Coletti, M. A., Sholl, D. S. & Allen-Dumas, M. R. Assessing impacts of atmospheric conditions on efficiency and siting of large-scale direct air capture facilities. JACS Au 4, 1883–1891 (2024).
-
Sendi, M., Bui, M., Mac Dowell, N. & Fennell, P. Geospatial analysis of regional climate impacts to accelerate cost-efficient direct air capture deployment. One Earth 5, 1153–1164 (2022).
-
Terlouw, T., Pokras, D., Becattini, V. & Mazzotti, M. Assessment of Potential and Techno-Economic Performance of Solid Sorbent Direct Air Capture with CO2 Storage in Europe. Environmental science & technology https://doi.org/10.1021/acs.est.3c10041. ObjectType-Article-1 (2024).
-
An, K., Farooqui, A. & McCoy, S. T. The impact of climate on solvent-based direct air capture systems. Appl. Energy 325, 119895 (2022). USDOE.
-
Perander, L., Chatzilamprou, I. & Klett, C. Increased Operational Flexibility in CFB Alumina Calcination, 49–53 (Springer International Publishing, Cham, 2016). https://doi.org/10.1007/978-3-319-48144-9_8.
-
Missalla, M., Klett, C. & Bligh, R. Design developments for fast ramp-up easy operation of new large calciners. TMS Light Metals (2007).
-
Hydro Québec. 2022 comparison of electricity prices in major North American cities: rates in effect April 1 https://www.hydroquebec.com/data/documents-donnees/pdf/comparison-electricity-prices.pdf (2022).
-
Environment and Climate Change Canada. Emission factors and reference values: Canada’s greenhouse gas offset credit system (2022).
-
McQueen, N., Desmond, M. J., Socolow, R. H., Psarras, P. & Wilcox, J. Natural gas vs. electricity for solvent-based direct air capture. Front. Clim. 2, 618644 (2021).
-
Environment and Climate Change Canada. Update to the pan-Canadian approach to carbon pollution pricing 2023-2030 (2021). https://www.canada.ca/en/environment-climate-change/services/climate-change/pricing-pollution-how-it-will-work/carbon-pollution-pricing-federal-benchmark-information/federal-benchmark-2023-2030.html
-
Arwa, E. O. & Schell, K. R. Batteries or silos: Optimizing storage capacity in direct air capture plants to maximize renewable energy use. Appl. Energy 355, 122345 (2024).
-
Wilcox, J.Carbon Capture (Springer Nature, New York, NY, 2012).
-
Wilcox, J., Rochana, P., Kirchofer, A., Glatz, G. & He, J. Revisiting film theory to consider approaches for enhanced solvent-process design for carbon capture. Energy Environ. Sci. 7, 1769–1785 (2014).
-
Onda, K., Sada, E. & Takeuchi, H. Gas absorption with chemical reaction in packed columns. J. Chem. Eng. Jpn. 1, 62–66 (1968).
-
Pinsent, B. R. W., Pearson, L. & Roughton, F. J. W. The kinetics of combination of carbon dioxide with hydroxide ions. Trans. Faraday Soc. 52, 1512 (1956).
-
Kucka, L., Kenig, E. Y. & Górak, A. Kinetics of the gas-liquid reaction between carbon dioxide and hydroxide ions. Ind. Eng. Chem. Res. 41, 5952–5957 (2002).
-
See, D. M. & White, R. E. Diaphragm cell measurement of mutual diffusion coefficients for potassium hydroxide in water from 1 °C to 25 °C. J. Chem. Eng. data 43, 986–988 (1998).
-
Bosma, J. C. & Wesselingh, J. A. Estimation of diffusion coefficients in dilute liquid mixtures. Chem. Eng. Res. Des. 77, 325–328 (1999). ObjectType-Article-2.
-
Smith, F. L. & Harvey, A. H. Avoid common pitfalls when using Henry’s Law. Chem. Eng. Prog. 103, 33–40 (2007).
-
Carroll, J. J., Slupsky, J. D. & Mather, A. E. The solubility of carbon dioxide in water at low pressure. J. Phys. Chem. Ref. Data 20, 1201–1209 (1991).
-
Sutherland, J. Analysis of mechanical-draught counterflow air/water cooling towers. Trans. ASME 105, 576–583 (1983).
-
Kuehn, T. H., Ramsey, J. W. & Threlkeld, J. L.Thermal environmental engineering (Prentice Hall, Upper Saddle River, N.J, 1998), 3rd edn.
-
American Society of Heating Refrigerating and Air-Conditioning Engineers Inc. 2017 ASHRAE Handbook – Fundamentals, chap. 6 (2017), si edn.
-
Strigle, R. F.Packed tower design and applications: random and structured packings (Gulf Pub. Co., Houston, 1994), 2nd edn.
-
Fogler, H. S. Elements of chemical reaction engineering. Prentice-Hall international series in the physical and chemical engineering sciences (Prentice Hall PTR, Upper Saddle River, NJ, 1997), 3rd ed. edn.
-
Schorcht, F., Kourti, I., Scalet, B. M., Roudier, S. & Delgado Sancho, L. Best available techniques (BAT) reference document for the production of cement, lime and magnesium oxide: Industrial Emissions Directive 2010/75/EU (integrated pollution prevention and control) 26129 (2013).
-
Hersbach, H. et al. Era5 hourly data on single levels from 1940 to present, https://doi.org/10.24381/cds.adbb2d47 (2023). Accessed: 2023-07-24.
-
Agustí-Panareda, A. et al. Technical note: The CAMS greenhouse gas reanalysis from 2003 to 2020. Atmos. Chem. Phys. 23, 3829–3859 (2023).
Acknowledgements
This research was undertaken with the financial support of the Government of Canada, and of Carleton University.
Author information
Authors and Affiliations
-
Department of Mechanical and Aerospace Engineering, Carleton University, Ottawa, ON, Canada
Patrick Shorey & Ahmed Abdulla
Contributions
Patrick Shorey: Methodology, Formal Analysis, Investigation, Writing – Original Draft, Writing – Review and Editing, Visualization. Ahmed Abdulla: Conceptualization, Methodology, Resources, Writing – Original Draft, Writing – Review and Editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Correspondence to Ahmed Abdulla.
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks David Yang Shu, ZHU Xuancan and Jan Wiegner for their contribution to the peer review of this work. Primary Handling Editors: Martina Grecequet. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.

Hi, this is a comment.
To get started with moderating, editing, and deleting comments, please visit the Comments screen in the dashboard.
Commenter avatars come from Gravatar.